Post-market clinical follow-up study report (PMCF study): Verification of the efficacy and safety of the medical device JETT PLASMA Medical II
Main Results
The primary efficacy endpoint was defined as an improvement of ≥ 1 point on the FWCS from baseline
at the 3-month follow-up after the last treatment. This improvement had to be confirmed by at least
two out of three blinded independent evaluators, based on the subject’s photographs. Based on the
primary endpoint, the response rate was calculated and analysed. The results are presented in Table
16.
Table 16 Analysis of the Primary Efficacy Endpoint:
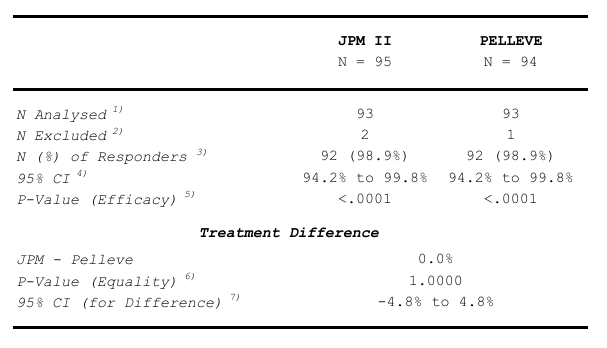
- The number of study subjects in the analysis population.
- The number of study subjects excluded from the analysis due to missing data of the variable.
- The number of responders and the response rate.
- 2-sided 95% confidence interval by treatment group (Wilson).
- H0: Response rate ≤ 0.75 vs. HA: Response rate > 0.75. One-sided.
- An auxiliary test for treatment inequality. It is not intended for the test of non-inferiority.
- 2-sided 95% confidence interval (Newcombe-Wilson) for the treatment difference. This interval is used for the test of non-inferiority.
In the analysis of the medical devices JPM II and Pelleve, a total of 95 subjects were initially enrolled in
the JPM II group, while 94 were in the Pelleve group (Table 16). However, due to missing data, the
number of subjects included in the primary analysis was slightly reduced, with 93 subjects analysed
from each group, because 2 and 1 subject were excluded from the JPM II treatment group and the
Pelleve group, respectively. Despite this, the analysis revealed a remarkably high response rate in both
groups, with 92 out of 93 subjects in each group classified as responders, translating to a response rate
of 98.9% for both JPM II and Pelleve.
The 95% CIs for the response rates presented in Table 16 were calculated using the Wilson method,
yielding intervals from 94.2% to 99.8% for both groups. This narrow confidence interval reflects the
precision of the estimated response rates.
A one-sided test was conducted to evaluate the efficacy of the treatments, with the null hypothesis
(H0) assuming a response rate of 75% (a pre-defined threshold). Because the resulting p-value was less
than 0.0001 for the JPM II treatment group, we could reject the null hypothesis of inefficacy of JPM II
at the α=0.025 level of significance one-sided, and conclude efficacy of the test product.
The same test was performed for the Pelleve group and it also could reject the null hypothesis of
inefficacy at the α=0.025 level of significance one-sided, and conclude efficacy of the Pelleve product.
This test had not been planned in the CIP. It may be used to support the idea that the study has assay
sensitivity.
Table 16 shows that the primary hypothesis of the inefficacy of JPM II was rejected in favour of its
efficacy. By maintaining the overall significance level at alpha=0.025 one-sided, it was then possible to
proceed to the non-inferiority test. This procedure followed the plan in the CIP regarding hierarchical
testing and was conducted in accordance with it. The results are also shown in Table 16. The 95% CI for
the treatment difference was from -4.8% to 4.8%. This confidence interval excluded the pre-defined
non-inferiority margin of -15% and lay entirely to the right of it. Therefore, at the α=0.025 level
significance one-sided, the null hypothesis of inferiority could be rejected and the non-inferiority of
JPM II concluded.
To assess the potential statistically significant difference in treatment effects between JPM II and
Pelleve, an auxiliary test for treatment equality was performed. The p-value for this test was 1.0000,
suggesting no significant difference was detected between the two devices in terms of their efficacy.
In summary, both JPM II and Pelleve demonstrated a high and practically identical response rate, with
statistical analysis confirming neither statistically nor clinically significant difference in efficacy
between the two medical devices.
See full study at: https://drive.google.com/file/d/1y9VhUmFg8EnXpaA_OVhnwP6BCoFJZ3Rf/view?usp=sharing or in the photos attached.
